- FRP Vessels
- New Compact Ball Valve
- Poly Glass Vessels
- Hydranautics RO Membrane
- Ion Pure EDI
- Pentair - Automatic Multiport Valve
- Initiative - Automatic Multi Port Valve
- FRP Membrance Housing
- SS Membrane Housing
- Pneumatic Actuated Ball Valve
- Purolite MZ10 Plus
- Ultrafiltration Membranes
- Aqualine Filter Cartridge
- Inline Rotameter
- Penal Mount Rotameter
- Filter Cartridge
- Filter Cartridge Housing
- Dosing Pump
- Filter Bag
- Filter Bag Housing
- Conductivity/TDS Meter
- Pressure Guage
- Activated Carbon
- Multi Port Valve (Manual)
- Distribution & Collection systems
- Industrial RO Controller
- 225NA ION Exchange Resin
- 225H Resin
- LABXT CEDI MODULES
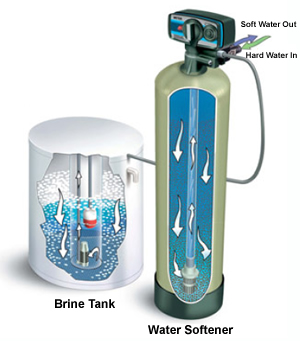
Water Softener
Hard water is frequently unsuitable for many industrial and domestic purpose. When water is referred to as 'Hard' it simply means, that it contains excess of calcium (Ca) and magnesium (Mg) Ions than normal water.
Application …. · Apartments / Condominiums · Hospitals · Restaurants · Institutions · Laundries · Boiler Feed Water · Food Processing Plants · Hotels · Office Complexes
· Residential Complexes |
|
|
|
|
| Top | |
| << Back Send Inquiry |